Dr John Lambert, PAREXEL.
Dr John Lambert, PAREXEL.
Proceedings from AHPPI’s conference on Risk Management in Early Phase Clinical Trials, 29 Oct 2015 (link).
John introduced his talk by describing the changing face of early phase clinical trials and the potential for ‘risk’ in studies that demonstrate an ever-increasing complexity in their design. The regulatory environment and requirements for risk evaluation and mitigation strategies were discussed.
John gave a fascinating insight into how PAREXEL, as a global CRO, addresses the various challenges. An insight was also given into the best way to navigate the regulatory and safety hurdles through a formal process of risk assessment for each and every study, with emphasis on being placed on first in human (FIH) studies.
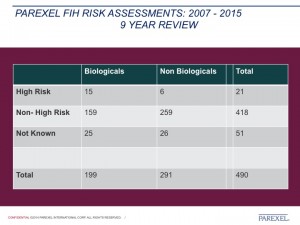
The second half of the presentation focused on risk mitigation strategies and how PAREXEL address the practical aspects of the design and staffing of FIH studies. John closed his presentation by sharing the risk assessment statistics on the number of FIH studies conducted by PAREXEL between 2007 and 2015. The talk stimulated a lively discussion between the speaker and the audience.
Written by Dr Tim Hardman. Tim is the Managing Director of Niche Science and
Technology Ltd., a CRO that has been working with industry and academia since 1998. Tim has a PhD from Imperial College, London, and degrees in Physiology and Pharmacology, along with over 20 years experience of developing new drugs. He is also a member of the AHPPI Secretariat.