Dr Richard FitzGerald, Royal Liverpool University Hospital.
Dr Richard FitzGerald, Royal Liverpool University Hospital.
Proceedings from AHPPI’s conference on Risk Management in Early Phase Clinical Trials, 29 Oct 2015 (link).
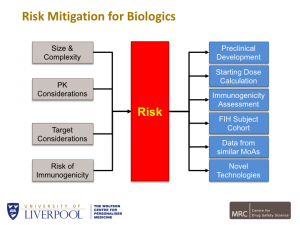
Starting with TeGenero and referring to subsequent guidelines for Phase I trials from such bodies as MRC, ABPI, expert scientific groups etc., Richard went on to describe the risks of clinical trials of various types of antibody, not all of which are monoclonal. He did, however refer specifically to mAbs, their therapeutic potential and the fact that several are currently marketed. With a comparison of small molecules and mAbs he argued that the latter may be safer due to being VERY target specific with NO off target effects and a simpler and predictable metabolism. However, by comparison, size and molecular complexity may lead to risk as there may be many target characteristics and expressions as well as targets which are cell based.
Potential immunogenicity has always been a risk and raises certain questions whereby preclinical programmes need to be optimized in order to support relevant clinical development especially with respect to integrated safety pharmacology: in terms of toxicology Richard showed the vast range of studies that have already been performed on marketed compounds, ranging from two weeks in mouse to 9 months in monkeys!
The risks of cytokine release syndrome were also stressed and this led on to consideration of the first dose to be given. The use of MABEL was stressed and it was pointed out that TGN 1412 has now been given safely to man in doses considerably lower than originally. Immunogenicity, cross reactivity and pre-existing antibodies were all discussed and the risks and benefits of healthy volunteers and patient groups explored with evidence from a meta analysis of first in man studies in an attempt to quantify risk. This was a wide-ranging and fascinating summary of the state of current knowledge.
Written by Dr Peter Dewland, Peter Dewland Consulting. Peter is an independent  consultant in pharmaceutical medicine and has a Master’s degree in Medical Ethics together with degrees in Biochemistry and Medicine along with 30 years experience of developing new drugs. He is a founder member of the Faculty of Pharmaceutical Medicine and now both teaches and examines students of this specialty. He is a past Chairman and current Treasurer of the AHPPI.
consultant in pharmaceutical medicine and has a Master’s degree in Medical Ethics together with degrees in Biochemistry and Medicine along with 30 years experience of developing new drugs. He is a founder member of the Faculty of Pharmaceutical Medicine and now both teaches and examines students of this specialty. He is a past Chairman and current Treasurer of the AHPPI.